Leukemia Translocation Panel
Available Products
| Product Name/Description | No. of Reactions* | Product Code |
| Leukemia Translocation Panel for Real-Time PCR | 24 | LEUKMP-RT24 |
*Includes all controls.
Fusion Genes and Leukemia
Many hematologic malignancies carry characteristic chromosomal translocations believed to play an important role in leukemia pathogenesis. Chromosomal translocations observed in leukemias fuse enhancer/promoter regions or different sequences of two separate genes. The result is aberrant expression, disruption of normal function of the native genes involved in the translocation, or gained oncogenic functions that promote proliferation and tumorigenesis.
Detecting and identifying fusion gene transcripts in patient blood or bone marrow samples enables accurate diagnosis, prognosis, and treatment stratification for ALL, APL, and AML. Following initial diagnosis, molecular monitoring of the individual fusion gene transcripts detected at diagnosis allows clinicians to initiate therapy at the point of molecular relapse, which has been demonstrated to be beneficial in comparison to therapy adjustment at clinical relapse.
The Leukemia Translocation Panel is a multiplexed, one-step RT-PCR assay that simultaneously detects 11 variants in six common translocations associated with ALL, AML, and APL. The panel also includes primer-probe sets to detect abl as a control gene. The assay is compatible with several leading multi-channel real-time PCR instruments and produces results in under 2 hours with only 15 minutes of hands-on time. One-step RT-PCR kits for individual fusion gene transcripts are available separately for molecular monitoring during treatment (see related products).
Assay Features
The Leukemia Translocation Panel provides reagents to detect and identify the fusion gene transcripts below in two, one-step reactions using total RNA isolated from blood or bone marrow at initial diagnosis:
| Disease | Translocations | Fusion Transcripts |
| ALL | t(1;19) | E2A/PBX1 (e13/e2) |
| t(12;21) | TEL/AML1 (e5/e2) | |
| t(4;11) | MLL/AF4 (e9/e5) | |
| MLL/AF4 (e10/e4) | ||
| APL | t(15;17) | PML/RARα (bcr1) |
| PML/RARα (bcr2) | ||
| PML/RARα (bcr3) | ||
| AML | Inv 16 | CBFB/MYH11 (A type) |
| CBFB/MYH11 (D type) | ||
| CBFB/MYH11 (E type) | ||
| t(8;21) | AML1/ETO (e5/e12) |
- One –step cDNA synthesis and quantification, eliminating the need to perform separate cDNA synthesis.
- Low contamination risk.
- Highly multiplexed reactions allow reference and target genes to be assessed in the same reaction.
- A rapid and efficient workflow. The panel may also be run concurrently with EntroGen’s BCR-ABL p210 and p190 assay for a comprehensive solution for initial leukemia diagnosis.
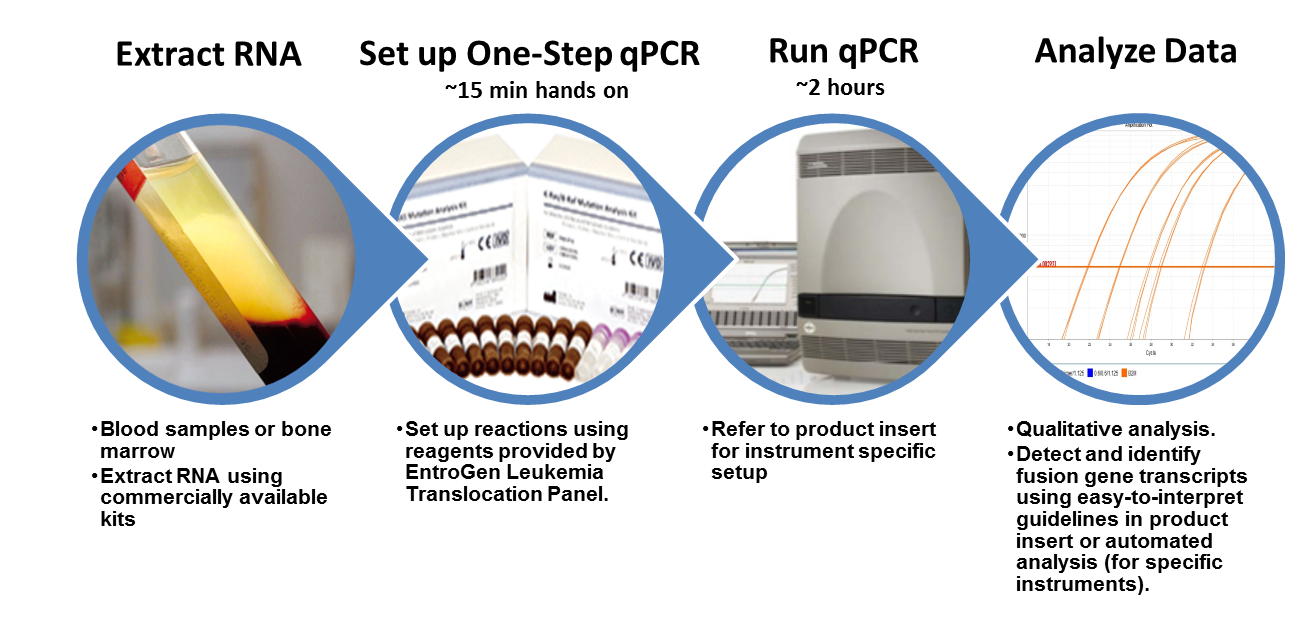
Workflow
Equipment and Materials
The Leukemia Translocation Panel requires a real-time PCR instrument capable of detecting FAM, VIC, ROX, and CY5 fluorescent probes. The panel includes reagents for cDNA synthesis and PCR amplification and detection. The panel also includes validated reaction controls.
The panel does not include reagents or columns for isolation of total RNA from blood or bone marrow.
Intended Use
USA: Available for research use only (RUO). Not for use in diagnostic procedures.
Europe: Available for research use only (RUO). Not for use in diagnostic procedures.
