Includes all controls.
Cell-free Mutation Detection
Tumor tissue from non-small cell lung cancer patients is routinely tested for the presence of somatic mutations in the epidermal growth factor receptor (EGFR) in order to predict response to EGFR tyrosine kinase inhibitor (TKI) therapy. However, in a significant number of cases, tumor tissue is not available in sufficient amount for accurate molecular testing. Recent studies have demonstrated the utility of cell-free tumor DNA (ctDNA) from plasma as an alternative source of genomic material for detection of sensitizing and resistance mutations in lung cancer.
EntroGen’s ctEGFR Mutation Detection Kit is a non-invasive, ultra-sensitive test to accurately detect the presence of EGFR activating mutations in ctDNA extracted from plasma. The test identifies the most common sensitizing (exon 19 deletions and L858R) and resistance (T790M) mutations in EGFR. Furthermore, quantitative calibrators enable quantification of absolute copy numbers of each variant in unknown specimens, making the test ideal for monitoring response over the course of therapy.
Testing Procedure
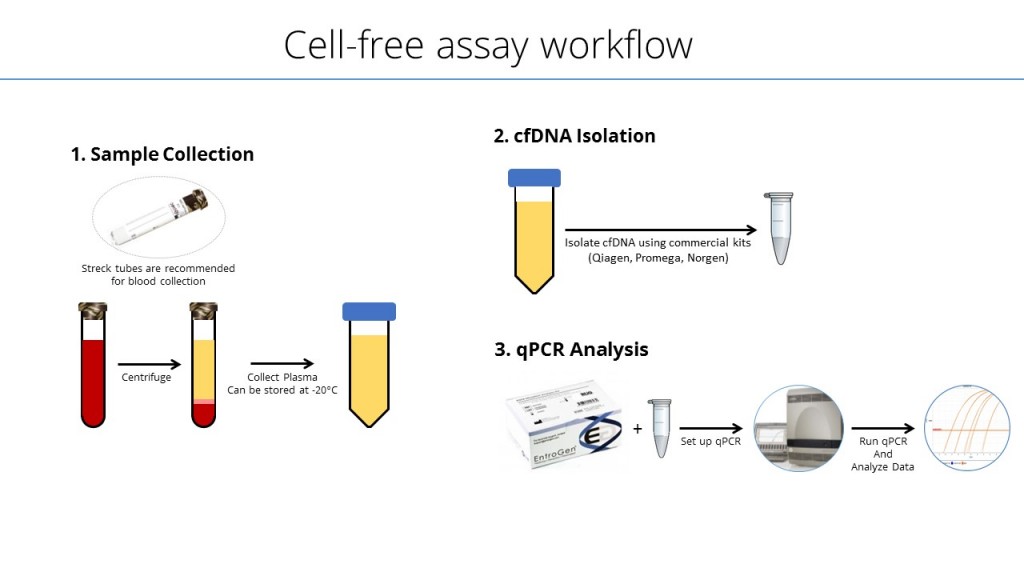
The ctEGFR Mutation Detection Kit is a real-time polymerase chain reaction (PCR)-based assay that uses mutant-specific primers to identify the presence of EGFR mutations. The testing procedure involves four (4) simple steps, which can be completed in approximately 2 hours from isolation of DNA to test results:
- Isolation of DNA from plasma
- PCR setup with reagents included in the kit
- Amplification and detection using a real-time PCR instrument
- Data analysis
Equipment and Materials
This kit requires a real-time PCR instrument capable of detecting FAM, VIC, ROX and CY5 fluorescent probes simultaneously.
This test includes reagents required for the PCR amplification/detection, as well as validated reaction controls. Columns and reagents for cell-free DNA isolation are not included.
Intended Use
USA: Available for research use only (RUO). Not for use in diagnostic procedures.
Europe: Available for research (RUO) and diagnostic (CE-IVD) purposes.
